Ivermectin: a Treatment that Deserves More Attention
By Tea Lynn
Ivermectin appears to be far more effective at treating COVID-19 than Remdesivir. So how is it that we’ve known about this possible miracle drug since early on in the pandemic and yet we are still not using it?
Ivermectin’s Story
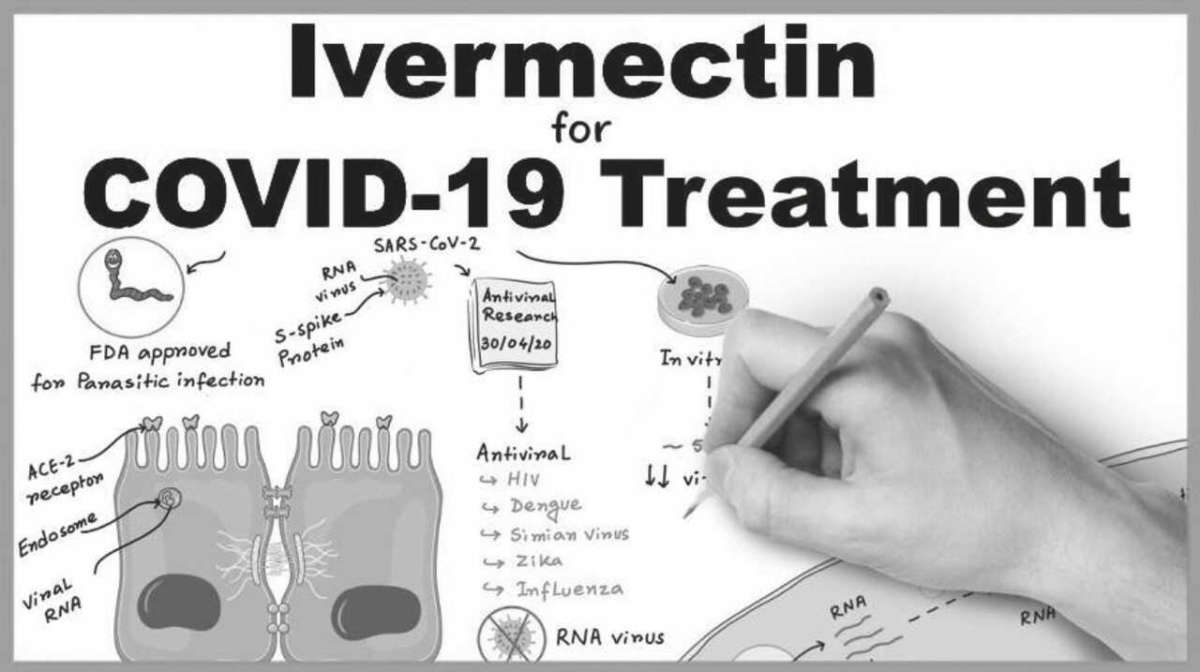
Back in late February, a Toronto nursing home had an outbreak of scabies. The treatment for scabies is the widely available drug Ivermectin. Just 1 pill lasts in the body for 28 days. The nursing home staff gave the pill to everyone on the afflicted floor. They also gave a smaller “preventative dose” to all other residents in the building. Little did they know the COVID-19 storm was just around the corner. Roughly 2 weeks later, COVID hit this Toronto nursing home in an unexpected way.
The floor that received the full dose of Ivermectin had no cases of COVID-19, despite the fact that that floor had the most amount of COVID-19 infected staff (who never took the drug). Throughout the nursing home’s 170 residents, only 6 caught COVID-19 and 4 of them were asymptomatic (including a 100-year-old woman). One resident died, but as described in an interview posted to Youtube, the daughter of the resident believes her death is unrelated to COVID-19.
Don’t lose touch with uncensored news! Join our mailing list today.
This is just one of many Ivermectin stories that entered the public consciousness in the spring of 2020.
Then, in early April, an in vitro study showed a single dose could reduce SARSCoV-2 by 93% in 24 hours, and eradicate it after 48 hours. After the release of that study, various attempts to have Health Canada study this drug were made to no avail by medical professionals.
Ivermectin was discovered in the 1970s and is on the World Health Organization’s list of essential medicines based on its versatility, affordability, safety and the beneficial impact that it has had, and continues to have, worldwide. The NIH classifies it as having a very high safety profile. Ivermectin is primarily thought of as an antiparasitic, but its anti-viral abilities have been detected in at least a dozen other viruses.
An Ivermectin tablet can cost as little as $2 – which could make it by far the cheapest, safest, and fastest cure for COVID-19 and the economy. Since just 1, 2$ pill lasts in the body for 28 days, this treatment could be administered at testing centres, for example, and there would be no fears of the patient doubledosing or forgetting to take their meds during the treatment period.
Internationally renowned Australian physician Dr. Thomas Borody, famously known for repurposing approved drugs to new treatments, is among the most outspoken advocate for the use of Ivermectin. “No trial has shown Ivermectin-based therapy to be ineffective. In-fact, international data reports an almost 100% cure rate and a symptom improvement within 4-6 days.”
Dr. Borody also recommends zinc and the antibiotic Doxycycline for patients with severe COVID.
Dr. Hibberd, a physician involved in a clinical trial with severe COVID stricken patients in Florida, reported that patients’ viral loads began declining almost immediately after they began administering ivermectin, “There’s a common denominator here,” he said “This drug is salvaging people from their death bed.”
By early June, various studies led to Ivermectin becoming widely popular in Latin America amongst the panic of raising cases. Local reports noted a run on pharmacies, draining their supply of Ivermectin. Some began using the veterinary formulation of the drug, instead of the one designed for humans. On June 22, the World Health Organization issued a statement saying that Ivermectin should not be used to treat COVID-19, and that it would not be included in its international Solidarity Trials, which included the antiviral drug Remdesivir and Hydroxychloroquine. The FDA also issued a warning not to selfmedicate with Ivermectin.
Despite all studies on Ivermectin showing extremely positive results, there seems to be a lack of interest among federal and global organizations.
Meanwhile, another medication has a completely different story.
Remdesivir’s Story
There’s a dirty little secret in scientific research: if you measure a large number of variables, throughout a number of studies, you are almost guaranteed to get a ‘statistically significant’ result in at least one study. This recipe for false positives (or exaggeration of true positives), known in the science world as “p-hacking” or “selective reporting,” seemed to be the method of choice for Gilead Science’s Remdesivir studies.
Gilead Science and their cronies have funded numerous Remdesivir studies this year. Most studies found little to no impact on the disease whatsoever. But they did have one (yes, just one ) good, well-designed study that actually seemed to show positive results. The results: Remdesivir has no superiority over the placebo in terms of mortality…. or at least that’s what the results should have stated if they hadn’t pulled out a few trial hacks.
When the study didn’t meet their expectations, they enrolled more people into the study to add different arms (to add more variables). They divided the patients up by severity (Who’s on regular flow oxygen? Who’s not? Who’s on high flow oxygen? Who’s on ECMO? Who’s on a vent?) and hoped that maybe the drug reduced mortality in at least one group… it did not.
They also retroactively changed the primary endpoint. Instead of seeing if the patients were alive and well on day 15, they instead looked at hospital stay length. They found that COVID-19 patients given Remdesivir had shorter hospital stay (from 15 to 10 days) … but this benefit was only seen in hospitalized patients sick enough to be receiving oxygen, but not sick enough to be receiving high flow oxygen, on a vent, or on ECMO.
Yes really. That was -still is- their best study. (And they make fun of Hydroxychloroquine -a drug that has over 200 studies with better results than this one).
This study was conducted by the NIH. Conveniently, of the 50 members on the NIH’s COVID-19 treatment panel at the time of this study, 9 had officially disclosed “conflicts of interests” with Gilead, which is the most amount of conflicts of interests by far for any one Pharmaceutical company. There are now 8 people on the panel tied to Gilead, as one member “lost their connection” somehow. T
he NIH has also invested billions of US dollars into Remdesivir research over the years. According to Ekaterina Cleary, lead data analyst and research associate at the Center for Integration of Science and Industry, “between gathering knowledge behind Remdesivir’s chemical structure and molecular target, the NIH invested as much as $6.5 billion between 2000 and 2019.”
Remdesivir is a very expensive drug. The cost of one 5-day course of treatment is a whopping $3,120 USD! Plus, since it is an IV medication, the patient has to be admitted to the hospital. In addition to the financial burden, Remdesivir treatment is not without risks. In late August the WHO noted a disproportionately high number of reports of liver and kidney problems in patients receiving Remdesivir compared with patients receiving other drugs for COVID-19. When studied in healthy volunteers prior to COVID-19, raised liver enzymes (a sign of liver damage) was a common side effect of the medication, seen in over 10% of volunteers.
On 15 October, the largest controlled study delivered what should have been the coup de grâce: The WHO’s Solidarity trial showed that Remdesivir does not reduce mortality or the time COVID-19 patients take to recover or the need for ventilation. The study, known as the Solidarity Trial, recruited almost 12,000 patients, making it the largest Covid-19 treatment study in the world thus far.
Despite the risks and lack of evidence of efficacy, the Food and Drug Administration gave its first full approval for a drug to treat Covid-19 to the antiviral Remdesivir on October 22, 2020. Marketed under the brand name Veklury, Remdesivir previously received emergency use authorization (EUA) from the FDA in May. Remdesivir was also the first drug that Health Canada authorized for the treatment of COVID-19, it was approved in July.
Full FDA and Health Canada approval promotes Remdesivir to the standard of care for hospitalized patients. Since the course of treatment requires hospitalization, this could unnecessarily lengthen total days spent in hospital for patients with milder cases of COVID-19.
Full approval also means it becomes much more difficult to conduct studies on other therapies because they now have to be compared against Remdesivir, the new standard treatment, as well as a placebo. This raises the cost and complexity of trials, delaying results.
It also makes it harder to recruit people for subsequent clinical trials. People may be more reluctant to sign up for a trial where they could get a placebo when they know they could get the actual drug.
The biggest, most serious problem is that we won’t get to the truth. There are now no more incentives to study whether Remdesivir works or not.
Why?
So why is it that expensive drugs showing little to no efficacy, like Remdesivir, is the standard of care for COVID-19 patients, yet more promising generic medications, like Ivermectin, are not even a consideration?
Answer: funding.
In order for a medication to be fully approved and promoted as the standard of care, it needs to have shown positive results in a well-designed clinical trial. Usually this means a large randomized double-blind placebo-controlled (RDBPC) study. And those are expensive.
Most clinical trials are funded by pharmaceutical companies with enormous financial stakes in the products being evaluated. Furthermore, the scientists who design, conduct, analyze, and report clinical trials often receive monetary compensation from drug companies, in the form of either salaries or consulting fees. And sometimes, particularly with the studies where funding is supplied by the pharmaceutical company themselves, their studies are designed to succeed.
Nobody wants to invest in medications that are cheap because they’re not money makers. So, most Ivermectin studies are “observational” – often times doctors taking it upon themselves to compare the patients prescribed Ivermectin to the patients who received standard care.
There have been several extremely successful randomized placebo-controlled studies, but it seems as though they are unfortunately being dismissed by authorities because they were not blinded and are coming out of countries like Egypt and Iraq.
One Ivermectin researcher, Dr. Sabine Hazan, got so fed up with not being able to obtain funding, that she invested hundreds of thousands of dollars or her own money into a clinical trial. Costs range from the medical equipment each patient will receive to monitor their improvement, such as Holter heart monitors and pulse oximeters, to the cost of hiring aides to draw blood regularly at patients’ homes.
Not even Hydroxychloroquine, perhaps the most talked about medication early on in the pandemic, has received the perfect study. HCQ is postulated to work in combination with zinc (as required for its mechanism of action) and only in early stage COVID-19 or as a prophylaxis (since it is thought to only work on the infection and not subsequent inflammation). Yet there has still never been a RDBPC study with Hydroxychloroquine and zinc in early stage COVID patients.
It’s hard to understand why, even amongst a global pandemic that has caused months-long lockdowns, the FDA or Health Canada or a large research institution wouldn’t fund an Ivermectin trial when it has showed such promise. In fact, it’s hard to understand why they wouldn’t fund a large handful of known antivirals at a time where billions upon billions of dollars are being spent on the pandemic response. How is it that only half a dozen anti-viral medications have been studied by both the WHO and the NIH combined?
The truth is any country could complete a study on whether or not Ivermectin helps COVID disease in the space of three weeks, and nobody’s done that. It’s worth one big, fast, well-designed, well-funded study and if I had my druthers, the government would make it so. We need to build a world where the government cares about the health of the people more than the health of the pharmaceutical industry.